Water vapour: feedback or forcing?
First some basics. Long-wave (or thermal) radiation is emitted from the surface of the planet and is largely absorbed in the atmosphere. Water vapour is the principle absorber of this radiation (and acknowledged as such by everybody). But exactly how important is it? In terms of mass, water vapour is much more prevalent (about 0.3% of atmospheric mass, compared to about 0.06% for CO2), and so is ~80% of all greenhouse gases by mass (~90% by volume). However, the radiative importance is less (since all molecules are not created equal). One way to quantify this is to take a radiation model and remove each long-wave absorber (principally the greenhouse gases, but also clouds and aerosols) and see what difference it makes to the amount of long-wave absorbed. This gives the minimum effect from each component. The complementary calculation, using only each particular absorber in turn, gives the maximum effect. Generally these will not be equal because of overlaps in the absorbing spectra (i.e. radiation at a particular frequency can either be absorbed by water vapour or CO2).
| Removed absorbers | Fraction LW | Rad. Forcing |
|---|---|---|
| absorbed | Tropo. (W/m2) | |
| None | 100% | 0 |
| H2O | 64 (64, RC78) | -56 |
| Clouds | 84 (86, RC78) | - |
| CO2 | 91 (88, RC78) | -23 |
| O3 | 97 (97, RC78) | |
| Other GHG | 98 | -3 |
| H2O+Clouds | 34 | - |
| H2O+CO2 | 47 | -89 |
| All except H2O+Clouds | 85 | - |
| All except H2O | 66 (60-70, IPCC90) | - |
| All except CO2 | 26 (25, IPCC90) | - |
| All except O3 | 7 | - |
| All except Other GHG | 8 | - |
| All | 0% | - |
| Instant calculation, global mean, Jan. 1, 1979 | RC78=Ramanathan and Coakley (1978) | |
| 'All' includes aerosols, O3 and other minor gases as additional absorbers. | ||
The table shows the instantaneous change in long-wave aborption when each component or combination of components is removed using the radiation code from the GISS GCM. (The source code is available for those who have the patience to get it to work). This isn't a perfect calculation but it's quick and easy and is close enough to the right answer for our purposes. (N.B. This is very similar to what was done by Ramanathan and Coakley (1978) using a single column model - their numbers are in the table for reference). Because of the overlaps, the combined changes are larger than the changes due to each individual component. Another calculation is the instantaneous radiative forcing at the tropopause, but that is complicated for clouds, O3 and Aerosols which have impacts on solar radiation as well as the long wave, so I only give that value for the 'pure' greenhouse gases.
The overlaps complicate things, but it's clear that water vapour is the single most important absorber (between 36% and 66% of the greenhouse effect), and together with clouds makes up between 66% and 85%. CO2 alone makes up between 9 and 26%, while the O3 and the other minor GHG absorbers consist of up to 7 and 8% of the effect, respectively. The remainders and uncertainties are associated with the overlaps which could be attributed in various ways that I'm not going to bother with here. Making some allowance (+/-5%) for the crudeness of my calculation, the maximum supportable number for the importance of water vapour alone is about 60-70% and for water plus clouds 80-90% of the present day greenhouse effect. (Of course, using the same approach, the maximum supportable number for CO2 is 20-30%, and since that adds up to more than 100%, there is a slight problem with such estimates!).
Since we are looking at the whole of the present-day greenhouse effect (around 33 C), it is not surprising that the radiative forcings are very large compared to those calculated for the changes in the forcing. The factor of ~2 greater importance for water vapour compared to CO2 is consistent with the first calculation.
So where does the oft quoted "98%" number come from? This proves to be a little difficult to track down. Richard Lindzen quoted it from the IPCC (1990) report in a 1991 QJRMS review* as being the effect of water vapour and stratiform clouds alone, with CO2 being less than 2%. However, after some fruitless searching I cannot find anything in the report to justify that (anyone?). The calculations here (and from other investigators) do not support such a large number and I find it particularly odd that Lindzen's estimate does not appear to allow for any overlap.
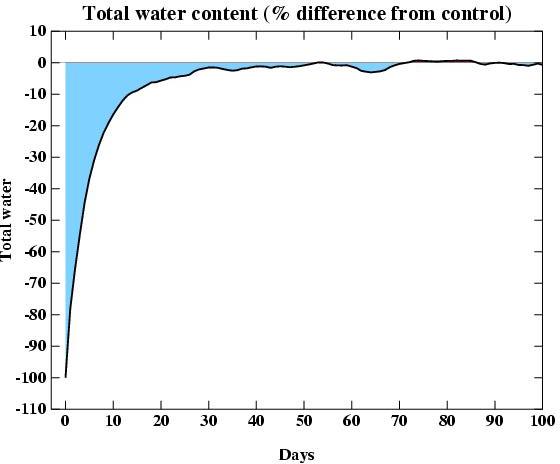
While water vapour is indeed the most important greenhouse gas, the
issue that makes it a feedback (rather than a forcing) is the relatively
short residence time for water in the atmosphere (around 10 days). To
demonstrate how quickly water reacts, I did a GCM experiment where I
removed all the water in the atmosphere and waited to see how quickly it
would fill up again (through evaporation from the ocean) . The result is
shown in the figure. It's not a very exciting graph because the
atmosphere fills up very quickly. At Day 0 there is zero water, but
after only 14 days, the water is back to 90% of its normal value, and
after 50 days it's back to within 1%. That's less than 3 months.
Compared to the residence time for perturbations to CO2
(decades to centuries) or CH4 (a decade), this is a really
short time.
Only the stratosphere is dry enough and with a long enough residence time (a few years) for the small anthropogenic inputs to be important. In this case (and in this case only) those additions can be considered a forcing. Oxidation of anthropogenic methane (which is a major source of stratospheric water) and, conceviably, direct deposition of water from increases in aircraft in the lower stratosphere, can increase stratospheric water and since that gives a radiative forcing effect, they do appear on the forcings bar chart (under "H2O from CH4"). Some scientists have argued that changes to irrigation and other land use changes (which effect evaporation) are also direct forcings to water vapour amounts, but I think it's cleaner to think of that as an indirect water vapour response to the change.
When surface temperatures change (whether from CO2 or solar forcing or volcanos etc.), you can therefore expect water vapour to adjust quickly to reflect that. To first approximation, the water vapour adjusts to maintain constant relative humidity. It's important to point out that this is a result of the models, not a built-in assumption. Since approximately constant relative humidity implies an increase in specific humidity for an increase in air temperatures, the total amount of water vapour will increase adding to the greenhouse trapping of long-wave radiation. This is the famed 'water vapour feedback'. A closer look reveals that for a warming (in the GISS model at least) relative humidity increases slightly in the tropics, and decreases at mid latitudes.
How do we know that the magnitude of this feedback is correctly simulated? A good test case is the response to the Pinatubo eruption. This caused cooling for up to 3 years after the eruption - plenty of time for water vapour to equilibriate to the cooler sea surface temperatures. Thus if models can simulate the observed decrease of water vapour at this time, it would be a good sign that they are basically correct. A good paper that demonstrated this was Soden et al (2002) (and the accompanying comment by Tony DelGenio). They found that using the observed volcanic aerosols as forcing the model produced very similar cooling to that observed. Moreover, the water vapour in the total column and in the upper troposphere decreased in line with satellite observations, and helped to increase the cooling by about 60% - in line with projections for increasing greenhouse gases.
To be sure there are still some lingering uncertainties. Some recent data indicates that tropical upper tropopsheric water vapour does not quite keep up with constant relative humidity (Minschwaner and Dessler, 2004) (though they still found that the feedback was positive). Moist convection schemes in models are constantly being refined, and it's possible that newer schemes will change things . However, given the Pinatubo results, the models are probably getting the broader picture reasonably correct.
*R.S. Lindzen, 1991. Quart. J. Roy. Met. Soc., 117, pp. 651-652
- So what do the models predict (or project) that
the additional water vapour contribution would be for a given rise in
temperature.
For example, if there was a change in GHG (WV not included) forcing which produced a temp rise of 1 deg C - by how much would that increase WV (in ppm) and what would that mean in terms of additional warming.
[Response: Since it's a coupled feedback process, you can't really separate it out like this. But you can estimate how much more water there would be for a given temperature increase at equilibirium using the Clausius-Clapeyron equation. For the response to 2xCO2, (around 3 deg C) you would expect an increase of about 30% in water vapour amounts. -gavin]
- OK - fair enough.
But I'm now getting a bit confused over the order of magnitude of the numbers involved. I thought - averaged globally - WV is measured in 1000s of ppm (i.e lots of 1000s in the tropics and much less at the poles). In your response, you say
For the response to 2xCO2, (around 3 deg C) you would expect an increase of about 30% in water vapour amounts
This means the increase is of the order of 300 ppm - or have I got something wrong.
[Response: The mean amount of water vapour in the atmosphere corresponds to about 25 mm of liquid water. At 2xCO2 that might increase to 32 mm or so. The change in ppm will vary a lot depending on where you are, but in the tropics, you might see changes of 1000s ppm (since the current surface values are ~16,000 ppm). - gavin]
- Let me see if I got it. We emit CO2, which warms
the world a bit, and this causes more water to vaporize & warm the world
more to a higher (more or less) stabilized level. And reducing
emissions, with nature aboring CO2 & atmospheric CO2 reducing, would
cool the earth a bit, which would reduce water vapor, cooling the world
more to a lower (more or less) stabilized level. So our emissions (or
reductions) have an somewhat amplified effect, beyond a purely CO2
effect.
And this scenario/model does not include the possibility of net positive feedback from nature (up to a much higher stabilized warming) - such as the warming (from CO2 and concomitant WV) reaching levels that cause nature to emit GHGs (from "natural" fires, decomposition, plant reduction, methane clathrates, albedo, etc.)
- Sounds cool to me Lynn, the critical line is the
one about residence time. It's not the quantity(state) of water vapour
that matters as much as the rate(residence time) of it's cycling. A
consequence of this is that water vapour is locally variable(15% - 100%
in the temperate zone) depending (mostly) on the wind direction and
season.
In terms of climate change this seems to lead to the sorts of conditions that are prevailing where I live in the south west of Australia . Here we experienced record flooding last week following an equinoctial storm. So that, although the winter rainfall has ben declining steadily since the 1960's, the average annual rainfall has been less affected due to increasing drought being offset by increasing storminess.
The deviation is as affected as much as the mean.
Comment by kyan gadac
- I'm not sure that serious skeptics worry too much
about the direct forcing from increased water vapor. What should be an
issue of discussion is how the increased water vapor plays out in terms
of increased cloud cover. As the now larger amount of water vapor rises
it will sooner or later result in cloud formation and this will, during
the day, cut off far more visible light than the amount of IR which is
blocked from escaping during the night. A good figure for what this
negative feedback from water vapor is must be used to offset the initial
positive feedback.
In this regard, it's also necessary to realize that the forcing from a given amount of additional CO2 or H2O decreases as the total amount of each increases. The rule of thumb I've seen is that each doubling of CO2 adds a constant forcing. Does that rule apply to water vapor as well?
Comment by Dave Dardinger
- Nice explanation of water vapor.
Question 1- when the long wave radiation is trapped by water vapor and CO2 etc How much heat energy is trapped? Do the models calculate this based on a concentration AND temperature/humidity dependant equation every 20-30 min cycle? I would assume that the GHG molecules do not saturate, ie trap the maximum amount of energy that they are capable of, just like the regular N2 and O2 molecules will not trap all the (smaller amount of)heat they are capable of, always leaving some room to heat up more as the solar flux gets hotter during the day, or during the season.Q2:Since the sun on a daily basis warms and cools the atmosphere by 20+ degrees (evaporating the morning dew/water vapor on the grass as it warms!) then how does this daily and variable cycling impact the residence time concept? ie Just how REAL is the water vapor residence time graph above? Wouldn't the water vapor readjust on the next daily cycle, and continue to adjust during the day as the daily temp and weather fluctuates?
Related question- Is there any daily cycling of CO2? Is the CO2 concentration temp dependant? Is the amount that dissolves in the nightly washed out water vapor trivial?
Just wondering!! Thanks.
[Response: If you look at the difference between the LW going out at the top (240 W/m2), and the LW emitted from the ground (380 W/m2), you get a 'trapped' portion of about 140 W/m2. There is a lot of absorbtion and re-radiation in the middle though. For some frequencies, the absorption is all very close to the ground, and they do saturate, but it varies strongly as a function of pressure and frequency. Another way to look at the residence time is to calculate the mean 'age' of water (how long it has been since that water parcel was evaporated). This gives very similar numbers to the gross calculation given above (i.e. around 10 days), but there are significant areas where the average age is longer (the stratosphere) and where it is shorter (near-surface in the tropics). And finally, for CO2, there is a daily cycling over vegetation (a few ppm), but the (very small) radiative effect is neglected in most models. -gavin]
- A hearty thanks to realclimate for doing an article on this. I can't tell you how many times I have had to explain the idea that, because of its short residence time, water vapor is not a greenhouse gas that we have much direct control over but is an important feedback effect. Now, I can just refer people here!
- What about a hydrogen economy in which we are
generating very high levels of water vapor, and some hydrogen leaks as
well. I read somewhere that this may have some negative impact, but I
can't remember.
Seems by your discussion (I'm not sure) that since the water vapor is close to the ground, only the amount ordained by the warmth of the climate will be held in the atmosphere, the rest precipitating away.
[Response: See Schultz et al (2003) or Warwick et al (2004) for discussion on the impact of a hydrogen economy. -gavin]
- In response to #12, as the temperature increases,
so does the capacity for air to retain moisture, so even if the quantity
of water vapour is to increase, relative humidities may not. In other
words, dewpoint depressions (T-Td) may remain the same.
Since RHs need to approach 100% (T-Td needs to approach zero) for clouds to form and since these RHs may not increase despite the aforementioned increases in water vapour, cloud cover may not increase, or if it does, it will not be significant.
[Response:To be really picky, air doesn't really "retain" moisture: the only thing of importance is the temperature. See http://www.atmos.umd.edu/~stevenb/vapor/ - William.]
- The important thing about water is, it is
saturated over most of the infrared spectrum. That means that 100% of
the infrared radiation emitted by the ground is absorbed by the water
vapor in the air. Whether there is more or less water vapor in the air
sometimes, it doesn't change the 100% absorption. That is what
saturation means.
Astronomers know a lot about this. It is why infrared telescopes are at extreme sites, on top of Mauna Kea or at the South Pole, where there is a lot lot lot less water vapor and so there is partial visibility at some infrared frequencies.
So the "greenhouse effect" caused by water vapor is a fixed constant not a dynamical variable, it doesn't change whether there is more or less water and so stick it into the equations and forget about it -- it doesn't change from century to century. Unless conditions got extreme, like widespread desertification on the one hand or permanent cloud cover on the other hand. (btw clouds contain liquid water not only vapor, so that's a different discussion.)
At other frequencies the water vapor does not absorb. The atmosphere is rather transparent at those frequencies. There is some partial absorption by CO2, but it is not saturated because there is much less CO2 in the atmosphere, compared to water vapor. If you put more CO2 into the atmosphere, radiation at those frequencies is absorbed proportionally more.
Note I made no mention of the residence time of either CO2 or H2O.
[Response: Parts of the infrared spectrum are saturated, parts are not. The effective forcing from any GHG change (including water vapour) needs to be intgerated over the entire spectrum. An instantaneous forcing calculation for 1.4xH2O over the whole globe gives a forcing of 5.5 W/m2 - demonstrating that over the whole spectrum, water is not saturated. - gavin]
Comment by Marc Feldman
That is indeed the case. The constant relative humidity would be produced as a result of calculations that the models make regarding evaporation of water from the surface and then of moving this water about through convection and the dynamics of the model [and raining the water out again].