RET™ technology addresses the above concerns. Many synthetic fuel processes do not fully utilize the entire energy released in the conversion steps. For example, in the production of electrical power, the internal energy of the waste steam leaving a turbine is condensed and the resulting heat is removed from the process through cooling. More than half of the primary source energy is lost through this one step alone. Another example is coal gasification, where air is a primary reactant in converting coal into useful synthesis gas. The product gases suffer from a lower heating value due to dilution of nitrogen from air that must be separated in order to increase the heating value of the product. Some of the energy is also lost from heating the nitrogen, which is considered an inefficient path for the released energy of the process. It is desirable to fully utilize as much of the generated energy in the conversion process as possible. How can this be accomplished? There are two basic ways that this can be done: (1) minimize the number of conversion steps, (2) fully utilize the internal energy of the product to minimize waste. RET™ technology uses both ways to improve yield and energy efficiency. Since any primary energy source can be used to drive RET™ technology, the technology is independent of the inherent physical and compositional characteristics of this source. Only heat is needed to drive RET™. Thus, geothermal, solar, or combustion of biomass or waste can be equally applied to produce hydrogen or chemicals. Since water vapor or steam is used as both an energy transfer medium as well as reactant for hydrogen production, the entire internal energy is utilized. RET™ also generates its own electrical energy in cracking water to hydrogen and oxygen. Since the technology is based on energy absorption, the internal energy of the waste steam is not wasted but contributes to the process of conversion to hydrogen.
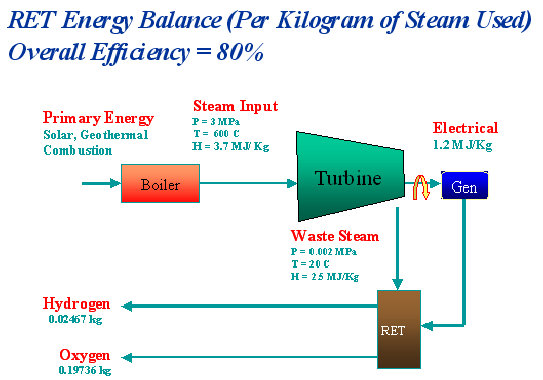
An energy balance is illustrated by clicking on the image above.
Thus, a kilogram of steam can produce about 0.02467 kilograms of hydrogen and 0.19736 kilograms of oxygen. In terms of volume produced, that amounts to about 276 liters of hydrogen and 138 liters of oxygen.