The Environmental Protection Agency (EPA) has just released its study on the Greenhouse Effect (Global Warming).
This is a synopsis of the report as presented.
Introduction
In June of 1992, the United States signed, and later ratified in October, the United Nations Framework Convention
on Climate Change (UNFCCC). The ultimate objective of the UNFCCC is "to achieve, in accordance with the relevant provisions of the Convention, stabilization of greenhouse gas concentrations in the atmosphere at a level that would prevent dangerous anthropogenic interference with the climate system. Such a level should be achieved within a time-frame sufficient to allow ecosystems to adapt naturally to climate change, to ensure that food production is not threatened and to enable economic development to proceed in a sustainable manner."2,3Intergovernmental Panel on Climate Change
(IPCC).The charter of the IPCC is to assess available scientific information on climate change, assess the environmental and socio-economic impacts of climate change, and formulate response strategies (IPCC 1996).
Greenhouse Gases
Although the Earth’s atmosphere consists mainly of oxygen and nitrogen, neither plays a significant role in
enhancing the greenhouse effect because both are essentially transparent to terrestrial radiation. The greenhouse effect is primarily a function of the concentration of water vapor, carbon dioxide (CO2), and other trace gases in the atmosphere that absorb the terrestrial radiation leaving the surface of the Earth (IPCC 1996).active gases and aerosols. We have clear evidence that human activities have affected concentrations, distributions and life cycles of these gases (IPCC 1996).Climate change can be driven by changes in the atmospheric concentrations of a number of radiatively
Naturally occurring greenhouse gases include water vapor, CO
2, methane (CH4), nitrous oxide (N2O), and ozone (O3).Water Vapor (H2O). Overall, the most abundant and dominant greenhouse gas in the atmosphere is water vapor. Human activities are not believed to affect directly the average global concentration of water vapor, but, the radiative forcing produced by the increased concentrations of other greenhouse gases may indirectly affect the hydrologic cycle.
Carbon Dioxide. In the atmosphere, carbon predominantly exists in its oxidized form as CO2. Atmospheric CO2 is part of this global carbon cycle, and therefore its fate is a complex function of geochemical and biological processes. ..The present atmospheric CO2 increase is caused by anthropogenic emissions of CO2" (IPCC 2001). The predominant source of anthropogenic CO2 emissions is the combustion of fossil fuels.
In its second assessment, the IPCC also stated that "[t]he increased amount of carbon dioxide [in the atmosphere] is
leading to climate change and will produce, on average, a global warming of the Earth’s surface because of its enhanced greenhouse effect.although the magnitude and significance of the effects are not fully resolved" (IPCC 1996).Methane
. Methane is primarily produced through anaerobic decomposition of organic matter in biological systems. Agricultural processes such as wetland rice cultivation, enteric fermentation in animals, and the decomposition of animal wastes emit CH4, as does the decomposition of municipal solid wastes. Methane is also emitted during the production and distribution of natural gas and petroleum, and is released as a by-product of coal mining and incomplete fossil fuel combustion. Atmospheric concentrations of CH4 have increased by about 150 percent since pre-industrial times, although the rate of increase has been declining. The IPCC has estimated that slightly more than half of the current CH4 flux to the atmosphere is anthropogenic, from human activities such as agriculture, fossil fuel use, and waste disposal (IPCC 2001).Nitrous Oxide.
Anthropogenic sources of N
2O emissions include agricultural soils, especially production of nitrogen-fixing crops and forages, the use of synthetic and manure fertilizers, and manure deposition by livestock; fossil fuel combustion, especially from mobile combustion; adipic (nylon) and nitric acid production; wastewater treatment and waste combustion; and biomass burning. The atmospheric concentration of N2O has increased by 17 percent since 1750,Ozone
. Ozone is present in both the upper stratosphere,9 where it shields the Earth from harmful levels of ultraviolet radiation, and at lower concentrations in the troposphere,10 where it is the main component of anthropogenic photochemical "smog." During the last two decades, emissions of anthropogenic chlorine and bromine-containing halocarbons, such as CFCs, have depleted stratospheric ozone concentrations.Halocarbons, Perfluorocarbons, and Sulfur Hexafluoride
. Halocarbons are, for the most part, man-made chemicals that have both direct and indirect radiative forcing effects. HFCs, PFCs, and SF6 are not ozone depleting substances, and therefore are not covered under the Montreal Protocol. They are, however, powerful greenhouse gases.Carbon Monoxide. Carbon monoxide has an indirect radiative forcing effect by elevating concentrations of CH4. Carbon monoxide is created when carbon-containing fuels are burned incompletely.
Nitrogen Oxides
. The primary climate change effects of nitrogen oxides (i.e., NO and NO2) are indirect and result from their role in promoting the formation of ozone in the troposphere and, to a lesser degree, lower stratosphere,Nonmethane Volatile Organic Compounds (NMVOCs)
. Nonmethane volatile organic compounds include substances such as propane, butane, and ethane. These compounds participate, along with NOx, in the formation of tropospheric ozone and other photochemical oxidants. NMVOCs are emitted primarily from transportation and industrial processes, as well as biomass burning and non-industrial consumption of organic solvents.Aerosols
. Aerosols are extremely small particles or liquid droplets found in the atmosphere. They can be produced by natural events such as dust storms and volcanic activity, or by anthropogenic processes such as fuel combustion and biomass burning.Greenhouse gases with relatively long atmospheric lifetimes (e.g., CO
2, CH4, N2O, HFCs, PFCs, and SF6) tend to be evenly distributed throughout the atmosphere, and consequently global average concentrations can be determined.The short-lived gases such as water vapor, carbon monoxide, tropospheric ozone, ozone precursors (e.g., NO
x, and NMVOCs), and tropospheric aerosols (e.g., SO2 products and carbonaceous particles), however, vary regionally, and consequently it is difficult to quantify their global radiative forcing impacts.Greenhouse Gas Emissions
Of course, here are the details of each. You'll need your ADOBE READER.
Agriculture
Agricultural activities contribute directly to emissions of greenhouse gases through a variety of processes. This
chapter provides an assessment of non-carbon dioxide emissions from the following source categories: enteric fermentation in domestic livestock, livestock manure management, rice cultivation, agricultural soil management, and field burning of agricultural residues.In 2002, agricultural activities were responsible for emissions of 467.1 Tg CO2 Eq., or 6.7 percent of total U.S.
greenhouse gas emissions. Methane (CH4) and nitrous oxide (N2O) were the primary greenhouse gases emitted by agricultural activities.
Energy
Energy-related activities, primarily fossil fuel combustion, accounted for the vast majority of U.S. CO
2 emissions for the period of 1990 through 2002. In 2002, approximately 86 percent of the energy consumed in the United States was produced through the combustion of fossil fuels. The remaining 14 percent came from other energy sources such as hydropower, biomass, nuclear, wind, and solar energy (see Figure ES-7 and Figure ES-8). A discussion of specific trends related to CO2 emissions from energy consumption is presented below.
Industrial Processes
Greenhouse gas emissions are produced as a by-product of various non-energy-related industrial activities. That is,
these emissions are produced from an industrial process itself and are not directly a result of energy consumed during the process. For example, raw materials can be chemically transformed from one state to another. This transformation can result in the release of greenhouse gases such as carbon dioxide (CO2), methane (CH4), or nitrous oxide (N2O).The processes addressed in this chapter include iron and steel production, cement production, ammonia manufacture and urea application, lime manufacture, limestone and dolomite use (e.g., flux stone, flue gas desulfurization, and glass manufacturing), soda ash production and use, titanium dioxide production, phosphoric acid production, ferroalloy production, CO2 consumption, aluminum production, petrochemical production, silicon carbide production, nitric acid production, and adipic acid production
In addition to the three greenhouse gases listed above, there are also industrial sources of several classes of manmade fluorinated compounds called hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6). The present contribution of these gases to the radiative forcing effect of all anthropogenic greenhouse gases is small; however, because of their extremely long lifetimes, many of them will continue to accumulate in the atmosphere as long as emissions continue.
Solvent and Other Product Use
Greenhouse gas emissions are produced as a by-product of various solvent and other product uses. In the United
States, solvent-related activities were a minor source of U.S. anthropogenic greenhouse gas emissions, accounting for less than 0.1 percent of total emissions on a carbon equivalent basis in 2002Waste
Waste management and treatment activities are sources of greenhouse gas emissions. Landfills
were the largest source of anthropogenic methane (CH4) emissions, accounting for 32 percent of total U.S. CH4 emissions. Smaller amounts of CH4 are emitted from wastewater systems by bacteria used in various treatment processes. Wastewater treatment systems are also a potentially significant source of nitrous oxide (N2O) emissions; however, methodologies are not currently available to develop a complete estimate. Nitrogen oxide (NOx), carbon monoxide (CO), and non-methane volatile organic compounds (NMVOCs) are emitted by waste activities,Trends in Greenhouse Gas Emissions
2.1. Recent Trends in U.S. Greenhouse Gas Emissions
As the largest source of U.S. greenhouse gas emissions, carbon dioxide (CO
2) from fossil fuel combustion has accounted for a nearly constant 80 percent of global warming potential (GWP) weighted emissions since 1990. Emissions from this source category grew by 17 percent.Historically, changes in emissions from fossil fuel combustion have been the
dominant factor affecting U.S. emission trends.Emissions from fuel combustion resumed a modest growth in 2002, slightly less than the average annual growth rate
since 1990. There were a number of reasons behind this increase.Partially offsetting this increased consumption of
fossil fuels, however, were increases in the use of nuclear and renewable fuels. Nuclear facilities operated at the highest capacity on record in 2002. Furthermore, there was a considerable increase in the use of hydroelectric power in 2002 after a very low output the previous year.
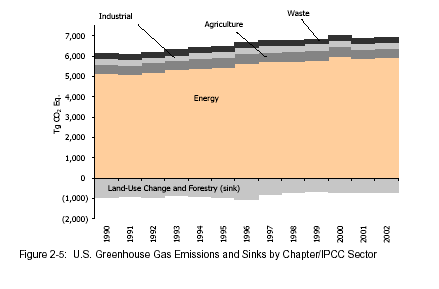
LOOK AT HOW MUCH THE PRODUCTION OF ENERGY (READ COMBUSTION OF FOSSIL FUEL) CONTRIBUTES TO GREENHOUSE EMISSIONS!!!
(Sorry, if the files below can't be opened, we're working on the problem!)
Or go to the EPA's website for the entire report and annexes: