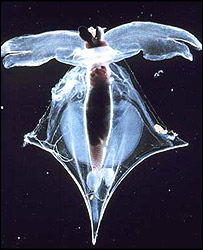
Shell of a live pteropod mollusc collected from the sub-arctic
Pacific
|
Consequently, atmospheric levels of the potent greenhouse gas are not nearly as high as they might have been.
But the heavy concentration of carbon dioxide in the oceans has changed their chemistry, making it hard for some marine animals to form shells.
The research is published in this week's edition of Science magazine.
Industrial age
Since the beginning of the industrial age around 1800, the amount of carbon dioxide (CO2) in the atmosphere has increased from 280 parts per million (ppm) to 380 ppm.
Although it seems a lot, many scientists were surprised: the extra CO2 that turned up in the atmosphere was only about half of the total amount emitted.
Following an international 10-year survey, researchers found the "missing" CO2 - it had been absorbed into the sea.
|
Christopher Sabine, NOAA
|
"If the ocean had not removed 118 billion metric tonnes of carbon between 1800 and 1994, the CO2 level in the atmosphere would be about 55 parts per million greater than currently observed."
Sea chemistry
This may have slowed global warming, but it also led to a change in seawater chemistry.
According to Richard Feely, of Noaa, and his colleagues, that might make life pretty hard for some shell-forming marine animals.
Corals, pteropod molluscs and some plankton (single celled organisms) pull carbonate ions from the seawater to produce their calcium carbonate shells.

The 10-year survey was an international effort
|
This means the animals lack the materials with which to build their shells.
And in areas where CO2 concentrations are particularly high, Professor Feely's team claim, the animal's shells can actually begin to dissolve.
"Based on our present knowledge, it appears that as seawater CO2 levels rise, the skeletal growth rates of calcareous plankton will be reduced - as a result of the effects of CO2 on calcification," said co-author Victoria Fabry, of California State University, US.
Patchy distribution
This effect is not witnessed uniformly throughout the oceans, however.
The survey has revealed that dissolved CO2 levels are rather patchy.
Because CO2 gets into the water by gas exchange at the surface - and because oceans tend to mix rather slowly - most CO2 is found near the top of the ocean, or in seas that are quite shallow.
"About half of the anthropogenic CO2 (i.e. produced by human activity) taken up over the last 200 years can be found in the upper 10% of the ocean," said Professor Sabine.

Sampling package used to collect water samples for the global
carbon dioxide survey
|
At the moment the oceans house a mere third of the CO2 that they could. Again, that is because our seas are "stirred" very slowly. So deep layers of water, which will eventually reach the surface, are far from being saturated.
"On the time scale of several thousand years, it is estimated that about 90% of the anthropogenic CO2 emissions will end up in the ocean," said Professor Sabine.
But the key word here is "thousands" of years. In the shorter term, as the surface waters become more saturated, the ocean may become a less efficient sink for CO2.
Just what that will mean for the Earth's climate, and for the marine ecosystem, is not quite clear yet.
Professor Sabine said: "Future studies of the carbon system in the oceans should be designed to identify and assess these feedback mechanisms [so that we can] determine the ocean's future role as a sink for CO2".