Quantum secrets of photosynthesis revealed
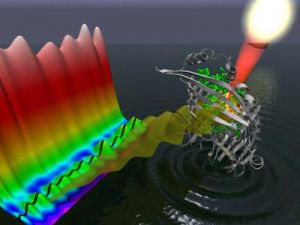
Sunlight absorbed by bacteriochlorophyll (green)
within the FMO protein (gray) generates a wavelike
motion of excitation energy whose quantum mechanical
properties can be mapped through the use of
two-dimensional electronic spectroscopy. Image
courtesy of Greg Engel, Lawrence Berkeley National
Laboratory, Physical Biosciences Division
A study led by researchers with the U.S. Department of
Energy’s Lawrence Berkeley National Laboratory (Berkeley
Lab) and the University of California (UC) at Berkeley
reports that the answer lies in quantum mechanical
effects. Results of the study are presented in the April
12, 2007 issue of the journal Nature.
"We have obtained the first direct evidence that remarkably long-lived wavelike electronic quantum coherence plays an important part in energy transfer processes during photosynthesis," said Graham Fleming, the principal investigator for the study. “This wavelike characteristic can explain the extreme efficiency of the energy transfer because it enables the system to simultaneously sample all the potential energy pathways and choose the most efficient one.”
Fleming is the Deputy Director of Berkeley Lab, a professor of chemistry at UC Berkeley, and an internationally acclaimed leader in spectroscopic studies of the photosynthetic process. In a paper entitled, Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems, he and his collaborators report the detection of “quantum beating” signals, coherent electronic oscillations in both donor and acceptor molecules, generated by light-induced energy excitations, like the ripples formed when stones are tossed into a pond.
Electronic spectroscopy measurements made on a femtosecond (millionths of a billionth of a second) time-scale showed these oscillations meeting and interfering constructively, forming wavelike motions of energy (superposition states) that can explore all potential energy pathways simultaneously and reversibly, meaning they can retreat from wrong pathways with no penalty. This finding contradicts the classical description of the photosynthetic energy transfer process as one in which excitation energy hops from light-capturing pigment molecules to reaction center molecules step-by-step down the molecular energy ladder.
"The classical hopping description of the energy transfer process is both inadequate and inaccurate," said Fleming. "It gives the wrong picture of how the process actually works, and misses a crucial aspect of the reason for the wonderful efficiency."
Co-authoring the Nature paper with Fleming were Gregory Engel, who was first author, Tessa Calhoun, Elizabeth Read, Tae-Kyu Ahn, Tomas Mancal and Yuan-Chung Cheng, all of whom held joint appointments with Berkeley Lab’s Physical Biosciences Division and the UC Berkeley Chemistry Department at the time of the study, plus Robert Blankenship, from the Washington University in St. Louis.
The photosynthetic technique for transferring energy from one molecular system to another should make any short-list of Mother Nature’s spectacular accomplishments. If we can learn enough to emulate this process, we might be able to create artificial versions of photosynthesis that would help us effectively tap into the sun as a clean, efficient, sustainable and carbon-neutral source of energy.
Towards this end, Fleming and his research group have developed a technique called two-dimensional electronic spectroscopy that enables them to follow the flow of light-induced excitation energy through molecular complexes with femtosecond temporal resolution. The technique involves sequentially flashing a sample with femtosecond pulses of light from three laser beams. A fourth beam is used as a local oscillator to amplify and detect the resulting spectroscopic signals as the excitation energy from the laser lights is transferred from one molecule to the next. (The excitation energy changes the way each molecule absorbs and emits light.)
Fleming has compared 2-D electronic spectroscopy to the technique used in the early super-heterodyne radios, where an incoming high frequency radio signal was converted by an oscillator to a lower frequency for more controllable amplification and better reception. In the case of 2-D electronic spectroscopy, scientists can track the transfer of energy between molecules that are coupled (connected) through their electronic and vibrational states in any photoactive system, macromolecular assembly or nanostructure.
Fleming and his group first described 2-D electronic spectroscopy in a 2005 Nature paper, when they used the technique to observe electronic couplings in the Fenna-Matthews-Olson (FMO) photosynthetic light-harvesting protein, a molecular complex in green sulphur bacteria.
Said Engel, "The 2005 paper was the first biological application of this technique, now we have used 2-D electronic spectroscopy to discover a new phenomenon in photosynthetic systems. While the possibility that photosynthetic energy transfer might involve quantum oscillations was first suggested more than 70 years ago, the wavelike motion of excitation energy had never been observed until now."
As in the 2005 paper, the FMO protein was again the
target. FMO is considered a model system for studying
photosynthetic energy transfer because it consists of
only seven pigment molecules and its chemistry has been
well characterized.
"To observe the quantum beats, 2-D spectra were taken at 33 population times, ranging from 0 to 660 femtoseconds," said Engel. "In these spectra, the lowest-energy exciton (a bound electron-hole pair formed when an incoming photon boosts an electron out of the valence energy band into the conduction band) gives rise to a diagonal peak near 825 nanometers that clearly oscillates. The associated cross-peak amplitude also appears to oscillate. Surprisingly, this quantum beating lasted the entire 660 femtoseconds."
Engel said the duration of the quantum beating signals was unexpected because the general scientific assumption had been that the electronic coherences responsible for such oscillations are rapidly destroyed.
"For this reason, the transfer of electronic coherence between excitons during relaxation has usually been ignored," Engel said. "By demonstrating that the energy transfer process does involve electronic coherence and that this coherence is much stronger than we would ever have expected, we have shown that the process can be much more efficient than the classical view could explain. However, we still don’t know to what degree photosynthesis benefits from these quantum effects."
Engel said one of the next steps for the Fleming group in this line of research will be to look at the effects of temperature changes on the photosynthetic energy transfer process. The results for this latest paper in Nature were obtained from FMO complexes kept at 77 Kelvin. The group will also be looking at broader bandwidths of energy using different colors of light pulses to map out everything that is going on, not just energy transfer. Ultimately, the idea is to gain a much better understanding how Nature not only transfers energy from one molecular system to another, but is also able to convert it into useful forms.
"Nature has had about 2.7 billion years to perfect photosynthesis, so there are huge lessons that remain for us to learn,” Engel said. “The results we’re reporting in this latest paper, however, at least give us a new way to think about the design of future artificial photosynthesis systems."
Source: Lawrence Berkeley National Laboratory
"We have obtained the first direct evidence that remarkably long-lived wavelike electronic quantum coherence plays an important part in energy transfer processes during photosynthesis," said Graham Fleming, the principal investigator for the study. “This wavelike characteristic can explain the extreme efficiency of the energy transfer because it enables the system to simultaneously sample all the potential energy pathways and choose the most efficient one.”
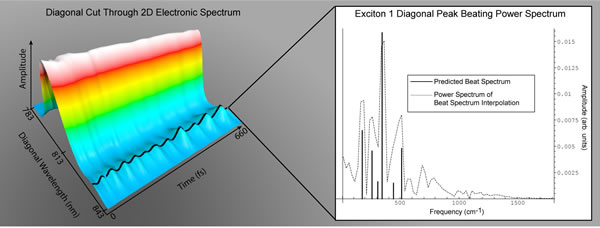 2-D electronic spectroscopy developed in the research group of Berkeley Lab’s Graham Fleming enables scientists to follow the flow of light-induced excitation energy through molecular complexes with femtosecond temporal resolution. In this 2-D electronic spectrum, the amplitude of the quantum beating signal for exciton 1 is plotted against population time. The black line covers the exciton 1 peak amplitude. The experimental data's agreement with theory is shown on the right. Credit: Lawrence Berkeley National Laboratory |
Fleming is the Deputy Director of Berkeley Lab, a professor of chemistry at UC Berkeley, and an internationally acclaimed leader in spectroscopic studies of the photosynthetic process. In a paper entitled, Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems, he and his collaborators report the detection of “quantum beating” signals, coherent electronic oscillations in both donor and acceptor molecules, generated by light-induced energy excitations, like the ripples formed when stones are tossed into a pond.
Electronic spectroscopy measurements made on a femtosecond (millionths of a billionth of a second) time-scale showed these oscillations meeting and interfering constructively, forming wavelike motions of energy (superposition states) that can explore all potential energy pathways simultaneously and reversibly, meaning they can retreat from wrong pathways with no penalty. This finding contradicts the classical description of the photosynthetic energy transfer process as one in which excitation energy hops from light-capturing pigment molecules to reaction center molecules step-by-step down the molecular energy ladder.
"The classical hopping description of the energy transfer process is both inadequate and inaccurate," said Fleming. "It gives the wrong picture of how the process actually works, and misses a crucial aspect of the reason for the wonderful efficiency."
Co-authoring the Nature paper with Fleming were Gregory Engel, who was first author, Tessa Calhoun, Elizabeth Read, Tae-Kyu Ahn, Tomas Mancal and Yuan-Chung Cheng, all of whom held joint appointments with Berkeley Lab’s Physical Biosciences Division and the UC Berkeley Chemistry Department at the time of the study, plus Robert Blankenship, from the Washington University in St. Louis.
The photosynthetic technique for transferring energy from one molecular system to another should make any short-list of Mother Nature’s spectacular accomplishments. If we can learn enough to emulate this process, we might be able to create artificial versions of photosynthesis that would help us effectively tap into the sun as a clean, efficient, sustainable and carbon-neutral source of energy.
Towards this end, Fleming and his research group have developed a technique called two-dimensional electronic spectroscopy that enables them to follow the flow of light-induced excitation energy through molecular complexes with femtosecond temporal resolution. The technique involves sequentially flashing a sample with femtosecond pulses of light from three laser beams. A fourth beam is used as a local oscillator to amplify and detect the resulting spectroscopic signals as the excitation energy from the laser lights is transferred from one molecule to the next. (The excitation energy changes the way each molecule absorbs and emits light.)
Fleming has compared 2-D electronic spectroscopy to the technique used in the early super-heterodyne radios, where an incoming high frequency radio signal was converted by an oscillator to a lower frequency for more controllable amplification and better reception. In the case of 2-D electronic spectroscopy, scientists can track the transfer of energy between molecules that are coupled (connected) through their electronic and vibrational states in any photoactive system, macromolecular assembly or nanostructure.
Fleming and his group first described 2-D electronic spectroscopy in a 2005 Nature paper, when they used the technique to observe electronic couplings in the Fenna-Matthews-Olson (FMO) photosynthetic light-harvesting protein, a molecular complex in green sulphur bacteria.
Said Engel, "The 2005 paper was the first biological application of this technique, now we have used 2-D electronic spectroscopy to discover a new phenomenon in photosynthetic systems. While the possibility that photosynthetic energy transfer might involve quantum oscillations was first suggested more than 70 years ago, the wavelike motion of excitation energy had never been observed until now."
"To observe the quantum beats, 2-D spectra were taken at 33 population times, ranging from 0 to 660 femtoseconds," said Engel. "In these spectra, the lowest-energy exciton (a bound electron-hole pair formed when an incoming photon boosts an electron out of the valence energy band into the conduction band) gives rise to a diagonal peak near 825 nanometers that clearly oscillates. The associated cross-peak amplitude also appears to oscillate. Surprisingly, this quantum beating lasted the entire 660 femtoseconds."
Engel said the duration of the quantum beating signals was unexpected because the general scientific assumption had been that the electronic coherences responsible for such oscillations are rapidly destroyed.
"For this reason, the transfer of electronic coherence between excitons during relaxation has usually been ignored," Engel said. "By demonstrating that the energy transfer process does involve electronic coherence and that this coherence is much stronger than we would ever have expected, we have shown that the process can be much more efficient than the classical view could explain. However, we still don’t know to what degree photosynthesis benefits from these quantum effects."
Engel said one of the next steps for the Fleming group in this line of research will be to look at the effects of temperature changes on the photosynthetic energy transfer process. The results for this latest paper in Nature were obtained from FMO complexes kept at 77 Kelvin. The group will also be looking at broader bandwidths of energy using different colors of light pulses to map out everything that is going on, not just energy transfer. Ultimately, the idea is to gain a much better understanding how Nature not only transfers energy from one molecular system to another, but is also able to convert it into useful forms.
"Nature has had about 2.7 billion years to perfect photosynthesis, so there are huge lessons that remain for us to learn,” Engel said. “The results we’re reporting in this latest paper, however, at least give us a new way to think about the design of future artificial photosynthesis systems."
Source: Lawrence Berkeley National Laboratory